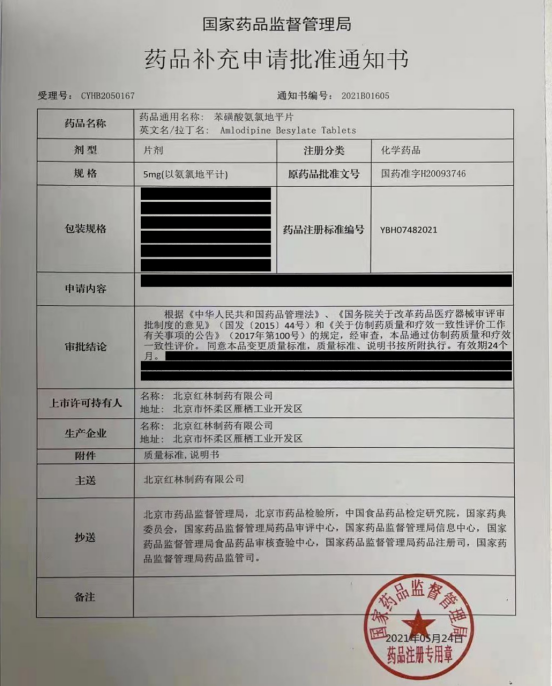
Beijing Honglin Pharmaceutical Ltd. Amlodipine Besylate Tablet passed the Generic Drug Consistency Evaluation!!
Recently, following the JUPOPINE® (Nefedipine Controlled-Release Tablets) & RoToFier® (Glipizide Extended Release Tablets), respectively, the National Medical Products Administration (NMPA) issued the certificate of Generic Drug Consistency Evaluation for Amlodipine Besylate Tablets to Beijing Honglin Pharmaceutical in response to the "Drug Application of Supplementary Amendments". This also makes Amlodipine the 3rd product of Honglin which passed the Generic Drug Consistency Evaluation.
Amlodipine Besylate Tablets follows the formula of Norvasc from Pfizer pharmaceuticals in USA, major targets are all types of hypertension and Coronary artery diseases. It's one of the highly recommended anti-hypertension drugs proposed by Official references. It is also able to be taken together with other kinds of medication. "1 pill per day" makes patients have better compliance.
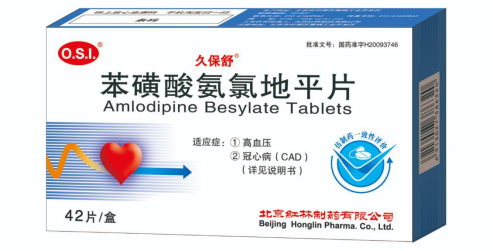
★ Generic Name
Amlodipine Besylate Tablets
★ Indications
1. Hypertension
2. Coronary artery disease "CAD" ( Details in the Users manuals )
★ Dosage and Administration
The initial dose of this product for the treatment of hypertension is usually 5mg once a day, and the maximum dosage is 10mg once a day. ( Please follow the Users manuals )
★ Package
5mg X 28/42/56 tablets.
★ Approval Number
H20093746
"Please take the drugs following the user manuals or under doctors’ prescription."

