Beijing Honglin Pharmaceutical Ltd. Nifedipine Controlled-release Tablets passed the Nifedipine Generic Drug Consistency Evaluation ! !
JUPOPINE® (Nifedipine Controlled-release Tablets), in 2010, due to the contemporary rules of naming system, was given the name of Nifedipine Extended-release Tablets III yet in fact it's Controlled-released. During the past 10 years, after series of training courses & promotions were given, deeply and widely admired by massive hypertension patients, it's now official named as "Nifedipine Controlled-release Tablets" and it passed the Nifedipine Generic Drug Consistency Evaluation.


JUPOPINE® (Nifedipine Controlled-release Tablets), with the adaptation of exclusive osmotic-pump technology and industrial manufacture techniques, entitlement of national patent and the cut-edge imported equipment, a higher product quality is guaranteed.
"Nifedipine Controlled-release Tablets" is overthrown by "Nifedipine Controlled-release Tablets" in terms of stability of drug release, avoidance of up-and-down of blood-drug density, steadiness of hypotensive effect, decrease of side effects, convenience of medication and patients compliances.
According to the "2018 modified version of National Anti-Hypertension References of China", prescription is subject to the effects of therapies and the long-term affordability of patients. Hypertension medication should start from small amount of dosage, priority should be given to long-term, combined treatment, and personal prescriptions.
Per daily dosage of "JUPOPINE® (Nifedipine Controlled-release Tablets)", its medical performance can last for 24 hrs, It also could be prescribed along with other kinds of hypertension drugs yet it has relatively higher Cost & Performance ratio than other kind of drugs with similar functions which totally complies the guidance of "2018 modified version of National Anti-Hypertension References".
In aspects of performances of medication and quality, "JUPOPINE® (Nifedipine Controlled-release Tablets)" in consistence to those of Bayer Adalat, specially adaptive to long-term hypertension patients, will become a great gospel for potential patients with chronicle diseases.
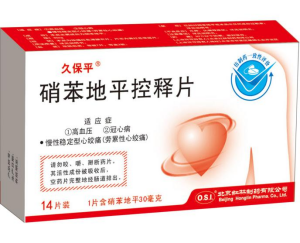
★ Generic Name
Nifedipine Controlled-release Tablets
★ Indications
1.Hypertension;
2.coronary heart disease
·Chronic Stable Angina (Classical Effort-Associated Angina)
★ Usage and Dosage
The administration for treatment shall be based on patient's individual case. The basic dosage of medication shall be different based on the clinical state of each patient. The medication of patients with liver dysfunction shall be carefully monitored, and the dosage of medication shall be reduced for severe cases.
The initial daily dosage for treatment is usually 30mg.
Unless there are special medical orders, the recommended dosage for an adult is: one tablet (30mg) once a day; the dosage can be increased to 60mg once a day if necessary.
Course of treatment: the medication time shall be decided by the doctor.
Usage of Drug: Swallow with small amount of liquid, no time constraints before or after meals.
★ Friendly Reminders
Tablets should be swallowed, can't be chewed, divided and smashed. Tablet like object could be visualized in the feaces, API is contained in an anti-dissolvable crust on the purpose of making the ingredients being stably and slowly released and being absorbed by the body of patients.
★ Package
30mg X 7/10/14/21/30 tablets.
★ Approval Number
H20103238
"Please take the drugs following the user manuals or under doctors'prescription."

