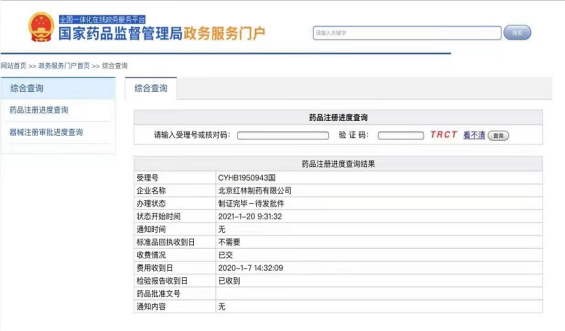
Honlin Glipizide Extended Release Tablets got the Generic Drugs Consistency Evaluation Certified!!
Beijing Honglin RoToFier® (Glipizide Extended Release Tablets), as the first manufacture who obtains the Certification of Glipizide Generic Drug Consistency Evaluation, applies the technology of osmotic-pump version controlled release, which makes possible the comparative stability of moderate API density in blood vessels by means of stable release of medical ingredients respectively minimizes the hypoglycemic incidence and meanwhile enhances the effect of the therapy.
Oral Hypoglycemic Medication is the effective therapy for type II diabetes, among them, insulin secretagogues is constantly the one of the referred medication from domestic and international authorities, among which Glipizide Extended Release Tablets Tablets is one of the classics.

★ Generic Name
Glipizide Extended Release Tablets
★ Indications
A drug as an auxiliary medication in addition to diet and exercise for the therapy of type II diabetes patients.
★ Usage and Dosage
Medication should be taken together with breakfast or the 1st meal of the day, recommended initial dosage is 5mg per dose and 1 dose a day.
★ Friendly Reminders
Tablets should be swallowed, can't be chewed, divided and smashed. Tablet like object could be visualized in the feaces, API is contained in an anti-dissolvable crust on the purpose of making the ingredients being stably and slowly released and being absorbed by the body of patients.
★ Package
5mg X 14/21/28 tablets.
★ Approval Number
H20084634
"Please take the drugs following the user manuals or under doctors’ prescription."

