2 Controlled Release Tablets of Beijing Honglin, earlier than others, are Consistency Evaluation Certified!!
---By MIC366
Recently, Beijing Honglin pharma.Co.,Ltd. ( Briefed as Beijing Honglin in the following text ), received 2 ratifications for the application of the "Supplementary Amendment of Medicine", one following another, from National Medical Products Administration (NMPA). Nefidipine and Glipizide Controlled Released Tablets of Beijing Honglin , being first Consistency Evaluation certified, respectively, justified themselves, among the others in China, the first 2 generic drugs whose quality and medical performance in consistent to those of the original drugs with controlled released production techniques.
In the ambience of encouragement of replacement of original drugs by generic drugs, the supply 2 controlled released tablets will incremental takes over the market share of the original drugs and brings gospels to mass patients with chronical diseases.
Advocating humanization of treatment, creating an optimizing combination of drugs and dosage forms
For patients with type II diabetes, even though the body can generates insulin, yet the cells can't make response which leads to a reduction of effectivity loss, this can be explained by that fact that due to the backfire of insulin system and the shortage of insulin secretion which result in the decrease of the usage ratio of the glucose in the body and in turns creates the symptoms of diabetes.
Conditions as above, oral hypoglycemic drug is one of the good options, among them insulin secretagogues is always the recommended medication of the domestic and overseas drugs directories, in which glipizide controlled-release tablets is one of the classic collection in the group of insulin secretagogues as it contains both the function of increase of insulin secretion and the moderation of the resistance of inslulin.
What's bothering the patients is that once taking glipizide normal tablet, not only patients may have to increase the times of medication, but also it comes along with adverse drug reactions, which is always a bottleneck for the cure of type II diabetes.
Against the challenge, after years of research, Beijing Honglin is proud to offer in the market "RoToFier(Glipizide Controlled-Release Tablet)". Compared to Glipizide normal tablet, in the duration of 24hrs after the drug taken, Glipizide Controlled-Release Tablets sustain effect drug density in blood, provide more stable blood glucose concentration, and generate less adverse drug reactions. in addition, the convenience of one-dosage-a-day increase the patient compliance.
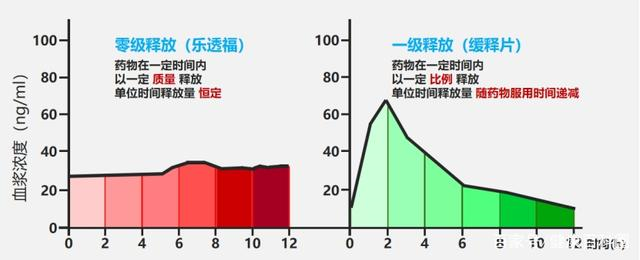
With Consistency Evaluation certified, RoToFier(Glipizide Controlled-Release Tablets)provide solutions for type II diabetes patients and make a big step forward in the course of anti-diabetes development in China.
Promotion of Stabilization of blood pressure decrease and Coexistence of "Convenience & Perfectness"
In terms of the patients with blood pressure, they also have similar perplex – multiple medication, vibration of blood pressure, high risk of cardiovascular disease.
According to the "China Anti-Hypertension Directions Modified Version in 2018" , small prescription is given in the start by doctors, and the guidelines of long-acting preparation, combined, individual-basis-treatment and drug economics are in priority, also the effectivity of therapies and the affordability of the patients are taken into consideration.
Nefidipine is one of the recommended medication against hypertension domestically and abroad, yet Nefidipine Extended-release tablets doesn't release the API at a consistent speed, in consequent it leads to the "more first and less later" release of drug ingredients in the body.
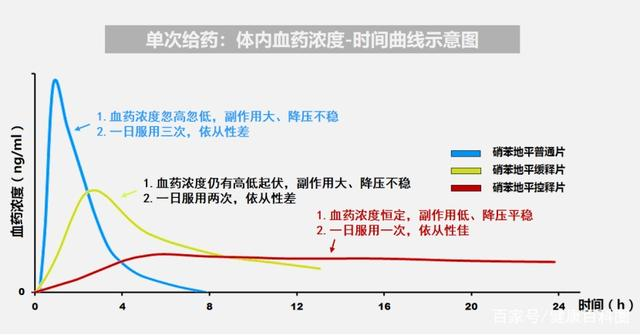
Comparatively, JUPOPINE Nefidipine Controlled-Release Tablets offers a more stable blood pressure control, more safety and conveniences by sustaining 24hrs of drug performance by taking once a day. Besides, it can also be taken together with other kinds of anti-hypertension drugs, also it offers a higher cost and performance ratio than other similar drugs in the market.
JUPOPINE, subject to the official naming rules in 2010, registration of JUPOPINE, osmotic-pump technology employed, was approved as "Nifedipine Extended-Released Tablets III" yet in fact it's a controlled-release tablet.By means of the latest Generic Drug Consistency Evaluation certification and the discussion in the joint-conference held by members in the committee of Pharmacopoeia of the People's Republic of China and China FDA, JUPOPINE was renamed as JUPOPINE Nifedipine Controlled Released Tablet in an anonymous agreement.
In more than 10 years of time, JUPOPINE has won the confidence of massive hypertension patient. Along with the certification of Generic Drugs Consistency Evaluation, Its significance is taken to a higher level.
With consistent & hard cultivation in a decade, Controlled-Released technology offers more options for patients.
RoToFier Glipzide & JUPOPINE Nefidipine are success stories of Beijing Honlin Pharmaceutical on treatment of hypertension and diabetes patients, basically they both share the same production technology – Osmotic-pump Controlled Released Technology.
Mechanism of dual layers osmotic pump controlled-released technology – after the drug is taken, water penetrates into the drug tablet core through the semi-permeable coating film, and the osmotic active substance inside the tablet core dissolves to generate a strong osmotic pressure. Driven by the osmotic pressure inside and outside the semi-permeable coating film and the expansion of the booster layer, the drug is released from the laser drug release hole slowly and at a constant zero-order rate.
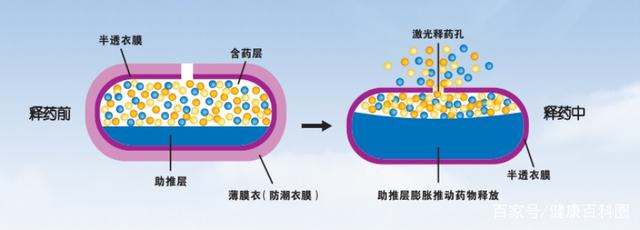
Since 1970, Alza company in USA first invented this osmotic pump technology, more than 10 products with the same technology was launched into the market. They are all drugs to be taken once a day.
Join-forced with Alza company for years, Beijing Honglin had developed a kind of technigue which manipulates the release of API, it overcomes the defect of the thermal instability and delay of drug release from the usage of traditional osmotic polymers.
The latest patented controlled-release technology, superior to that of the older generation, has better quality and most flexible storage requirement.
As a top level drug manufacturer, Beijing Honglin keeps its commitment by investing more in R&D and the exploration of the filed of controlled-release technology. Honglin pharmaceutical will also keep all options open for the cooperation opportunities, domestic & abroad to help more patients in China!

