“Heavy Weight” Generic Drugs from prestigious pharmaceutical are Consistency Evaluation Certified!!
---By MIC366
01 // Original Drugs receiving challenges in a 8.6 billion market
Recently, in the latest announcement released by NMPA, many famous drugs receive the certification of Generic Drug Consistency Evaluation. Among them is JUPOPINE® - Nefidipine Extended-release Tablets (Ⅲ) .
Worthy to be mentioned, dated back to 2010, according to the contemporary naming rules, JUPOPINE was approved to be launched into the market as class Ⅲ Extended-release Tablets.
Along with this certification of Generic Drugs Consistency Evaluation and the discussion in the joint-conference held by members in the committee of Pharmacopoeia of the P.R.C and NMPA, JUPOPINE® - Nefidipine Extended-release Tablets (Ⅲ) is officially named as the Nefidepine Controlled-Release Tablets which makes Beijing Honglin the first company with the product justified having the same quality level and therapeutic performance as the original drug.
Allegedly, Nifedipine Controlled-release Tablets was co-developed both by Beijing Honglin & Alza Pharmaceutical Ltd. in USA.
With the technical support given by Alza, the "Founding Father" of controlled-release technology all along the course of development, following the pharmaceutical formula and the adapting the osmotic-pump controlled-release technology of Adalat from Bayer Germany, equipped with edge-cutting production facilities imported from US & Europe, Beijing Honglin enforce high quality control in rigid level on the production of JUPOPINE.
Based on the statistics of Menet Co,.Ltd., in 2020, the potential market scale of Nefidipine in the clinical system could go up to 6.8 billion, on the top of that, Market scale of OTC market accounts for 1.8 billion. Either one, the market scale of Nifidipine is huge, undoubtedly.
Graph 1 : Growth of the market scale of Nefidipine in China Clinacal system. (unit of 10 thousand)
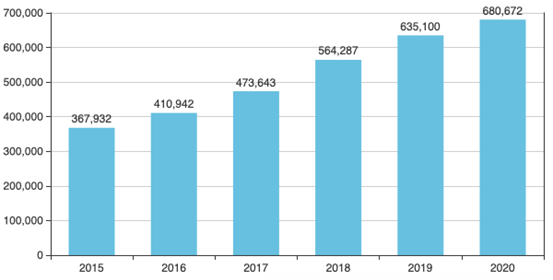
(Source of Data : Menet)
Being first Conformance Evaluation certified, quality level and medical performance justified consistent to those of the original, JUPOPINE will incremental takes over the market share of the original drugs and brings impacts to the market of anti-hypertension drugs in the ambience of encouragement of replacement of original drugs by generic drugs from government.
02 // Nefidipine, highly recommended for Clinical therapies in Medicine Directories.
Nefidipine, the most applied in the therapy of CCB (Calcium Channel Blocker) anti-hypertension drug, is the top 1 recommended in the domestic and oversea directories for drug usage.
Based on the statistics of the Menet, the market size of CCB related anti-hypertension drug amounts to 20 billion, yet Nifidipine already gets ahead of Amloidipine, and becomes the top 1 favored drug.
Graph 2 : Pie Chart of Top 20 CCB relative anti-hypertension drugs in 2020
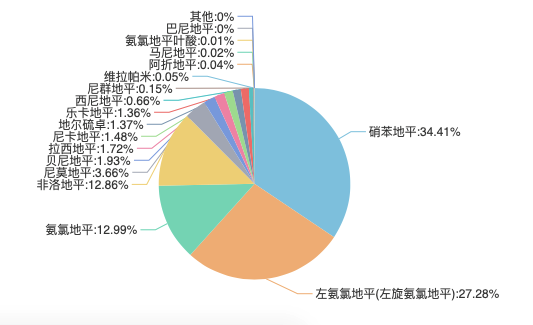
(Source of Data : Menet)
Currently, in view of the Adverse Drug Reaction (ADR), in clinical practices the mainstream usages of Nefidipine are Controlled & Extended Released Tablets. However, the speed of drug release of Controlled released tablets is slower and steadier than that of extended release, the opportunity of ADR is smaller.
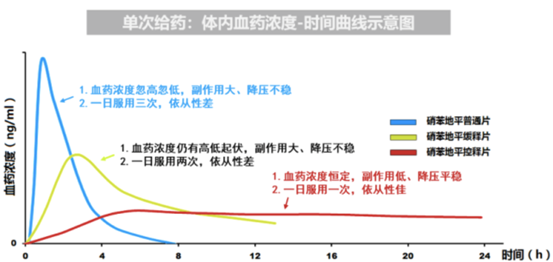
Either in the field of API or finishing product, market of Nefidipine is always highly competitive. The technology of Controlled Released Technology will hence become the "Holy Grail" for the victory in the competitive market, it is also the technology which sustain the comparative advantage of Adalat in decades.
Mechanism of Dual Layers osmotic-pump controlled released technology as follow. After the tablet taken, water in the body will be pushed into the inside of the tablet through the membrane and react with the chemical substances, following by a strong osmotic pressure generated from the dissolving matters and the outgrowing pressure derived from the push layer, API is pushed out from the drug layer through the hole opened by the laser driller into the patient’s body at a slower and steady speed. That is why the drug release is not influenced by the intermediate, PH value, enzymes, enterogastric peristalsis, and digestion of food also there is no constraint of meal schedule for the medication.
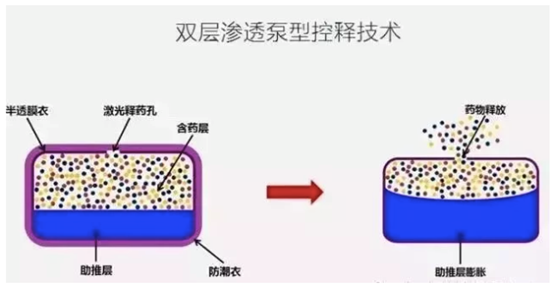
Therefore, in more than 10 years of time, JUPOPINE has won the confidence of massive diabetes patients by means of the controlled release technology. Along with the certification of Conformance Evaluation, its significance in the course of anti-hypertension drug and the service to the clinical system is taken to a higher level.
03 // A Market Interested by Various Pharmaceutical Companies.
According to the latest investigation implemented by China Hypertension Annual Conference, currently there are already 0.3 billion of patients with high-blood pressure diseases, and an increasing number of 100 million per year, worst of all, it’s getting rejuvenating.
Proven by the facts above, market demand of drugs for hypertension disorder is huge. But indicated by clinical therapists, a big number of patients have the mentality of resistance to medication, poor disciplines of medication, medication upon emergencies.
According to the "China Anti-Hypertension Directions Modified Version in 2018" Small prescription is given in the start by doctors, and the guidelines of long-term, combined, individual-basis-treatment and drug economics are in priority, and the effectivity of therapies as well as the affordability of the patients should be taken into consideration.
Obviously, as a long-term medication for anti-hypertension in clinical treatment, Controlled Released tablets has overwhelming advantages. After the tablet is taken, the effectivity of JUPOPINE can sustain in a duration of 24hrs, and it can be taken in combination with other kinds of anti-hypertension drugs. Besides, it provides advantages of avoidance of up and down of blood-medicine density, steadier blood pressure, less ADR, longer duration of drug effectivity, convenience of medication and better patients compliances.
In terms of the advantages of Nefedipine Controlled Released Tablet such as high level of market recognition, higher C/P value over Oral Normal & Extended Release tablets, numbers of players are focusing this market.
Based upon an investigation from Menet, nowadays there are 9 new players in to this market while 1 player is in the process of application of Conformance Evaluaiotn.
Regarding to domestic pharmaceutical manufactures, once breakthrough the "technology barrier" and get conformance evaluation certified, bigger chances they will have to take over the existing market of Adalat Bayer. That is also the fundamental factor why more and more players in giving their focus in this market.

